Technology
How a lateral flow test works
Rapid immunochromatographic tests (also known as lateral flow assays) were originally developed in the late 1980s as a fast, simple means of testing for pregnancy. Since then, this method, which requires neither specialist staff nor special laboratory equipment, has gained wide acceptance for a variety of point-of-care and field-use applications in human, veterinary and consumer diagnostics. The main components of a lateral flow assay and the assay formats used are described below.
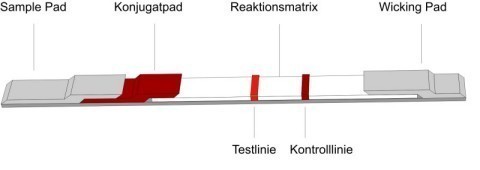
The image above shows the typical design of a lateral flow assay. It consists of a sample and conjugate pad, a nitrocellulose membrane and a wicking pad. The individual components of the test strip overlap each other and are affixed to a plastic backing.
The sample is first applied to the sample pad, where it is modified to make it compatible with the other test components.
Special whole blood separators may be incorporated in the sample pad to ensure that only serum reaches the second test component, the conjugate pad, where a particulate conjugate has been immobilised. The latter generally consists of gold particles, but latex is also often used. The particles are linked to the first biological component of the assay (an antibody or antigen), and as the sample migrates to the conjugate pad, it rehydrates the conjugate, thus allowing a reaction to take place between the analyte and the biological component linked to the gold particles. This complex then migrates to the third component of the assay, the reaction matrix. The reaction matrix typically consists of a nitrocellulose membrane to which the other specific biological components of the assay have been applied.
The test line typically consists of proteins (antigens or antibodies) which capture the primary analyte-antibody complex and generate a colour signal. Conjugated particles that have not bound to the test-line bind to the control line, where in most cases a species-specific immunoglobulin, which reacts specifically with the antibody conjugated to the colloidal gold, is immobilised.
Thus a colour signal is generated at the control line irrespective of the result at the test line. The fourth component of the lateral flow assay is the wicking pad. It adsorbs the fluid from the nitrocellulose membrane and acts as the motor of the whole assay.
The sample formats used in lateral flow assays are either direct (sandwich immunoassay) or competitive (inhibition immunoassay) and allow qualitative, semi-quantitative and, in certain cases quantitative analysis of the analyte.
These direct rapid test systems are used for detecting large analytes with multiple antigenic determinants, such as the Parvovirus that can be detected using our Fassisi ParCo test kit. In this case, a positive result is indicated by the appearance of a test line and a control line.
The following film demonstrates how a sandwich immunoassay works. As an analyte is present in the sample both the test and control line appear in the assay.

Contact
Fassisi, Gesellschaft für Veterinärdiagnostik und Umweltanalysen mbH
Marie-Curie-Strasse 8
37079 Göttingen
Germany
info@fassisi.de
Phone: +49 (0)551 5008840





